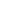
Pluslife Released HPV Molecular Tests with High-Risk Genotypes (16/18/45) for Cervical Cancer Screening
- Categories:News & Events
- Author:
- Origin:
- Time of issue:2022-11-14 15:55
- Views:
(Summary description)
Pluslife Released HPV Molecular Tests with High-Risk Genotypes (16/18/45) for Cervical Cancer Screening
(Summary description)
- Categories:News & Events
- Author:
- Origin:
- Time of issue:2022-11-14 15:55
- Views:
Cervical cancer is one of the global burdens that ranks as the fourth most common cancer among women. With 99% of cervical cancer cases caused by high-risk human papillomaviruses (HPV) infections, a Global Strategy towards the Elimination of Cervical Cancer as a Public Health Problem was initiated by WHO in 2019. In line with WHO’s determination, Pluslife has rolled out HPV 16/18/45 test to the global market and partnered with FIND, the strategic partner of WHO devoted to implementing the Next Generation POC Molecular System in low- and middle-income countries, areas where cervicalcancerhasdouble incidence rates and triple death rates than high-income countries.
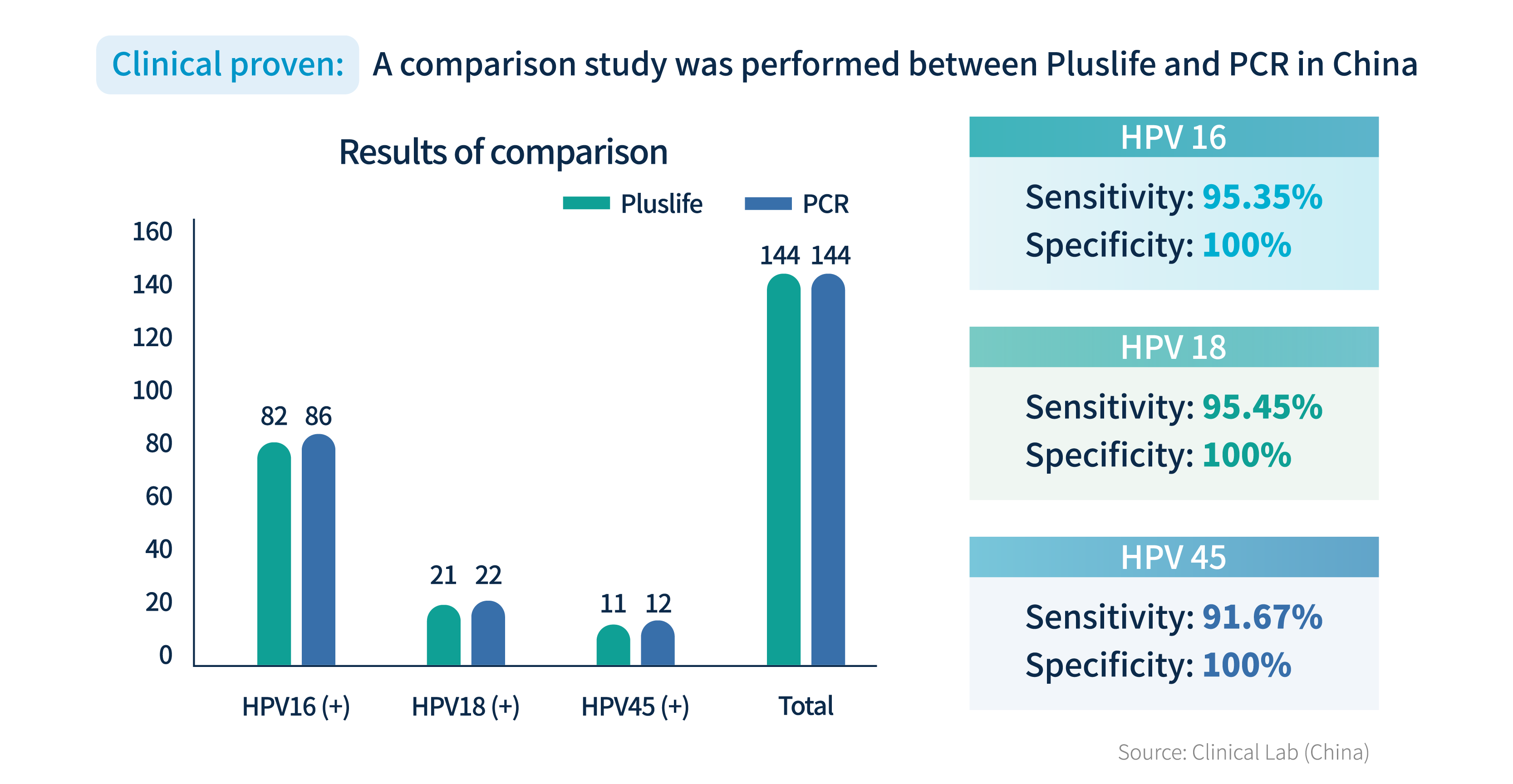
While over one hundred HPV genotypes had been discovered, certain genotypes have higher attributions to cervical cancer. HPV 16 and 18 together are responsible for more than 70% of cervical cancer, followed by HPV 45 which is responsible for 6%. However, cervical cancer is a preventable yet curable disease, using Pluslife HPV 16/18/45 Test to implement screening in high-risk individuals allows early detection and effective treatment to be made.
According to WHO, for women in general, HPV DNA Nucleic Acid Amplification Tests (NAATs) are the recommended test for primary screening of cervical cancer, beginning at age 30 with a 5 to 10 years screening interval. However,research showed that current cervical cancer testing programs are varied between nations and medical facilities (hospitals/clinics/GP/others), it is suggested that existing cervical testing programs using cytology tests or Visual Inspection with Acetic Acid (VIA) asthe primary screening method should be transited to NAATs by WHO.
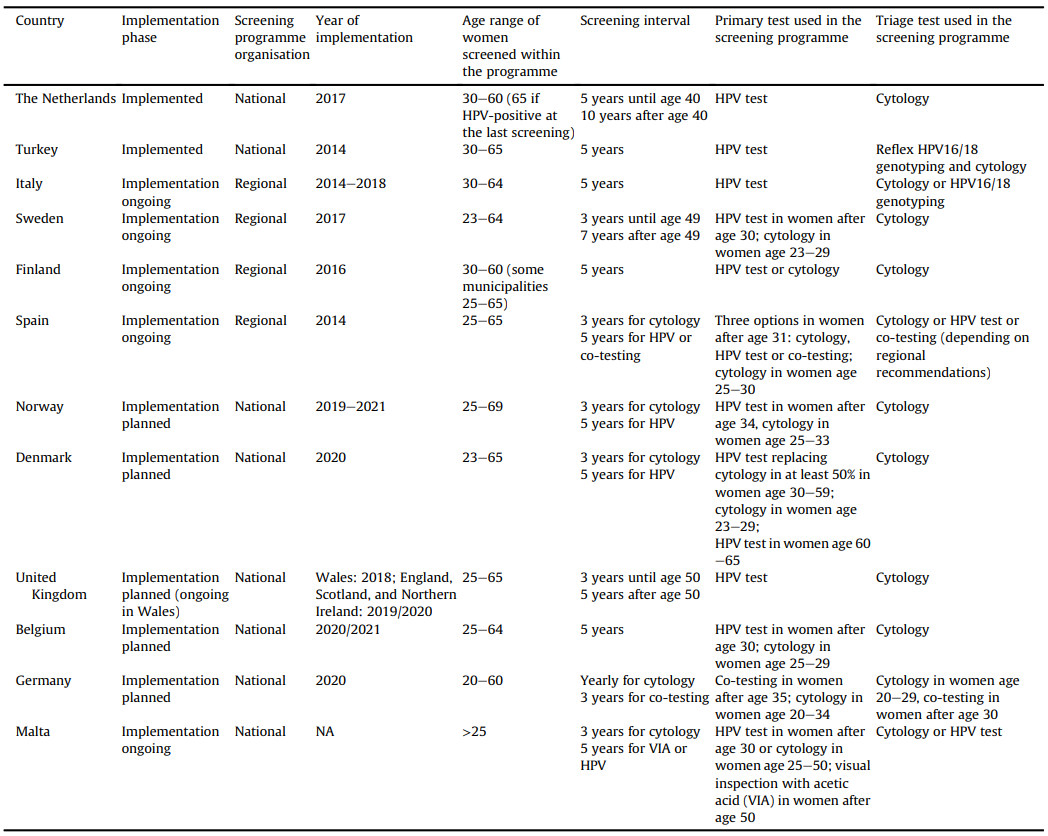
(Table: Summary of current status of implementation of HPV-based cervical cancer screening in selected European countries and main characteristics of the screening programmes)
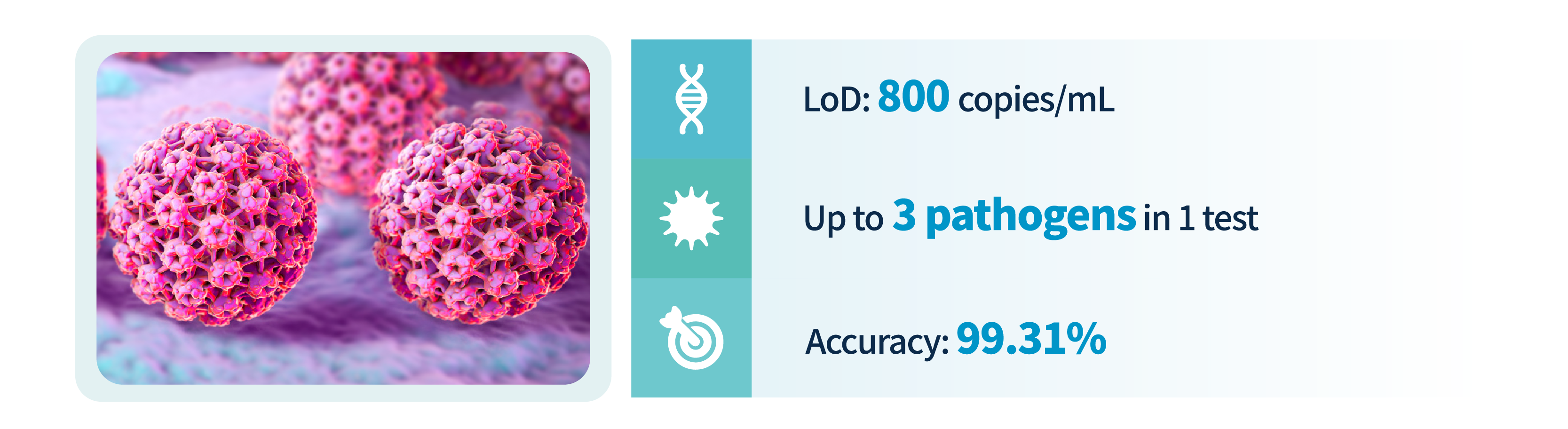
Toacceleratethetransitionofcervicalcancertestingprogramsindifferentmedical facilities (hospitals/clinics/GP/others), Pluslife HPV16/18/45 Test asa DNA-based molecular NAAT with excellent flexibility can make this iteration happenssimply. Pluslife HPV16/18/45 Tests works together with thepalm-sized MiniDock Molecular POCT System that requires no complex infrastructure and highly trained personnel, leading the way for the possibility of self-testing that allows more regular participation in screening for patients who concern withpersonal privacy issues.With the POCT feature, test results can be released on-site as fast as 35 minutes. Meaning a more efficient patient management could be achieved in comparison with the traditional cytologic tests that normally take more than 24 hours for results. Most importantly, extensive evidence had confirmed higher sensitivity and accuracy in HPV DNA tests than in traditional cytologic tests, doctors and clinicians can make confident diagnostic decisions through the use of Pluslife HPV16/18/45 Test with a proactive “screen, triage and treat” approach.

Pluslife HPV16/18/45 Nucleic Acid Test Card
(Isothermal Application Chip Method)
Jointly use with Pluslife Mini Dock Molecular POCT System to accelerate patient management efficiency with fast and accurate 3-in-1 results in 35 mins.
Reference:
- Maver, P. J., & Poljak, M. (2020). Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans.Clinical microbiology and infection,26(5), 579-583.
- Cuzick, J., Clavel, C., Petry, K. U., Meijer, C. J., Hoyer, H., Ratnam, S., Szarewski, A., Birembaut, P., Kulasingam, S., Sasieni, P., & Iftner, T. (2006). Overview of the European and North American studies on HPV testing in primary cervical cancer screening.International journal of cancer,119(5), 1095–1101. https://doi.org/10.1002/ijc.21955
- Rompalo, A. M., Hsieh, Y. H., Hogan, T., Barnes, M., Jett-Goheen, M., Huppert, J. S., & Gaydos, C. A. (2013). Point-of-care tests for sexually transmissible infections: what do 'end users' want?.Sexual health,10(6), 541–545. https://doi.org/10.1071/SH13047
- de Sanjose S, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010 Nov;11(11):1048-56.
- file:///D:/%E8%BD%AF%E4%BB%B6/9789240014107-eng%20(1).pdf
Tel: +86-20-31703986
E-mail:
(Business Enquiry)
(After-Sales&Technical Support)
Add: 3F-Block E, Runhui Science & Technology Park, No. 18 Shenzhou RD, Huangpu District, Guangzhou, Guangdong, China
Copyright © 2021 Guangzhou Pluslife Technology Co., Ltd. 粤ICP备19038432号


 +86-2031607074
+86-2031607074 Consulting
Consulting